
Abdullah Ilhan1; Umit Yolcu1; Emin Oztas2; Uzeyir Erdem3
DOI: 10.5935/0004-2749.20180045
ABSTRACT
Purpose: Nitrogen mustard (NM) is a devastating casualty agent in chemical warfare. There is no effective antidote to treat NM-induced ocular injury. We aimed to assess the effects of proanthocyanidin (PAC) and coenzyme Q10 (CoQ10) on NM-induced ocular injury.
Methods: Eighteen male rats were divided into the following 4 groups: NM, NM + PAC, NM + CoQ10, and control. The 3 NM groups received a single dose of NM (0.02 mg/µL) on the right eye to induce ocular injury. The control group received saline only. Thirty minutes after the application of NM, the NM + PAC group received PAC (100 mg/kg) via gastric gavage, while the NM + CoQ10 group received CoQ10 (10 mg/kg) via intraperitoneal injection. PAC and CoQ10 were administered once a day for 5 consecutive days. The rats were then sacrificed. Macroscopic images of the eyes were examined and eye tissues were collected for histology.
Results: The treatment groups were compared to the control group with regard to both corneal opacity and lid injury scores. The findings were not significantly different for both the NM + PAC and NM + CoQ10 groups. In both the NM + PAC and NM + CoQ10 groups, the histological changes seen in the NM group demonstrated improvement.
Conclusions: Our results indicate that PAC and CoQ10 treatments have therapeutic effects on NM-induced ocular injury in a rat model. PAC and CoQ10 may be novel options in patients with NM-induced ocular injury.
Keywords: Mustard gas; Eye injuries; Oxidative stress; Proanthocyanidins; Ubiquinone
RESUMO
Objetivo: A mostarda de nitrogênio (MN) é um agente de guerra química devastador. Não existe um antídoto eficaz para tratar lesões oculares induzidas por MN. Nosso objetivo foi avaliar os efeitos da proantocianidina (PAC) e da coenzima Q10 (CoQ10) na lesão ocular induzida por MN.
Métodos: Dezoito ratos machos foram divididos em 4 grupos: MN, MN + PAC, MN + CoQ10 e Controle. Três grupos receberam uma dose única de MN (0,02 mg/µL) destilada no olho direito para gerar lesão ocular. Os animais do grupo controle receberam apenas solução salina. Trinta minutos após a aplicação de MN nos animais, o grupo MN + PAC recebeu PAC (100 mg/kg) por gavagem gástrica, enquanto a CoQ10 (10 mg/kg) foi administrada ao grupo MN + CoQ10 por meio de injeção intraperitoneal. A administração de PAC e de CoQ10 foi realizada uma vez por dia, durante 5 dias consecutivos. Os ratos foram, então, sacrificados. Imagens macroscópicas dos olhos foram examinadas e tecidos oculares foram coletados para histologia.
Resultados: Os grupos de tratamento foram comparados ao grupo de controle quanto à opacidade da córnea e quanto aos escores de lesão da cobertura da córnea. Os resultados foram insignificantes para ambos os grupos. Ambos, o grupo MN +PAC e o grupo MN+CoQ10, apresentaram melhoras das alterações histológicas observadas no grupo MN.
Conclusões: Nossos resultados indicam que os tratamentos com PAC e com CoQ10 têm efeitos terapêuticos sobre lesões oculares induzidas por MN em um modelo em ratos. A proantocianidina e a CoQ10 podem ser uma nova opção nesses casos.
Descritores: Gás de mostarda; Traumatismos oculares; Estresse oxidativo; Proantocianidinas; Ubiquinona
INTRODUCTION
Mustard agents are some of the most devastating casualty agents in chemical warfare, and they have been used since World War 1 in at least 12 conflicts(1). They are considered the first option in chemical warfare because of their simple and inexpensive manufacturing approaches(2). The primary aim of using mustard agents is not to cause sudden death but to incapacitate opponents through painful burns, mucosal injuries, respiratory insufficiencies, ocular surface irritation, and secondary infections, which require tiresome and long-lasting medical treatments, resulting in complete occupation of medical facilities with hundreds of chronically affected patients and an increase in the economic burden.
Nitrogen mustard (NM) belongs to the group of mustard agents referred to as vesicant agents that especially affect the skin, eyes, and lungs after exposure(3). The degrees of inflammation and ocular tissue damage depend on the duration and dosage of exposure. After exposure to mustard agents, ocular injury symptoms occur in 1-12 h. Common symptoms include eyelid burns, severe eye pain, photophobia, excessive lacrimation, and visual deterioration(4,5). Corneal epithelium loss occurs, and sometimes, total epithelial detachment is noted in a few hours.
Although there are numerous treatment options for mustard agent-induced injuries, there is no effective antidote to prevent or treat NM-induced ocular injury. Rapid decontamination to stop further exposure is considered essential(6). Additionally, eyes should be irrigated with water or decontamination fluids(7). Local anti-inflammatory and antibiotic drugs, such as doxycycline, dexamethasone, and diclofenac, can be helpful in treatment(2,89). Despite these efforts, ocular injury remains a serious issue that needs to be addressed along with other systemic complications.
Oxidative stress plays a significant role in the pathogenesis of several acute and chronic diseases, including ocular disorders(10). Recent studies have shown that oxidative stress and inflammatory responses, including inducible nitric oxide, cyclooxygenase-2, and matrix metalloproteinase-9 induction; nuclear factor kappa-B (NF-κB) activation; and oxidative DNA damage, play vital roles in mustard agent-induced skin injury(11). Mustard agent-induced ocular injury has been reported to show inflammatory histological changes in the cornea(8).
Coenzyme Q10 (CoQ10) is a coenzyme for inner mitochondrial enzyme complexes involved in ATP production, and it is endogenously synthesized in mammals. It has been reported to have strong antioxidant properties(12). Coenzyme Q10 also prevents lipid peroxidation and DNA damage induced by oxidative stress(13,14). These properties are associated with its free radical-scavenging activity(15).
Flavonoids are natural phenolic compounds and well-known antioxidants(16,17). Proanthocyanidin (PAC), a polyphenol flavonoid isolated from grape seeds, has antioxidant effects and free radical-scavenging activity, and it is a derivative of flavan-3-ol flavonoids. The antiinflammatory effects and antioxidant properties of PAC have attracted significant interest in the treatment of several diseases(18,19).
The potential value of antioxidant treatment for ocular diseases has created a new area of interest for ophthalmologists. In NM-induced ocular injury, PAC and CoQ10 may suppress and regulate intraocular oxidative stress and inflammation. However, the efficacy of PAC and CoQ10 for NM-induced ocular injury is unclear. Thus, the present experimental study aimed to determine the efficacy of PAC and CoQ10 for NM-induced ocular injury.
METHODS
Animals
Eighteen male Sprague-Dawley rats (weight, 200-250 g) were obtained from the Health Sciences Institute, Gulhane Military Medical Academy (Ankara, Turkey). They were provided food and water ad libitum and were handled humanely, according to principles set out by the Association for Research in Vision and Ophthalmology. This study was conducted in the Research Center of Gulhane Military Medical Academy upon receiving approval from the Animal Ethics Committee of Gulhane Military Medical Academy.
Experimental design
All animals were randomly divided into the following 4 groups: NM (n=5), NM + PAC (n=5), NM + CoQ10 (n=5), and control (n=3). PAC (grape seed oil), CoQ10, and NM (mechlorethamine) were purchased from Sigma-Aldrich Co. (LLC, Laborchemikalien GmbH Seelze, Germany). The NM + PAC and NM + CoQ10 groups were treatment groups. Rats in the study groups (NM, NM + PAC, and NM + CoQ10 groups) were deeply anesthetized with intraperitoneal (i.p.) injections of ketamine (100 mg/kg) and xylazine (10 mg/kg). After induction of anesthesia, 5 µL of NM (0.02 mg/µl in balanced saline solution [BSS]) was applied in a single dose to the central cornea of the right eye to induce ocular injury. In the control group, 5 µL of BSS only was applied. We performed a pilot study to validate the NM dose and quantity for this study. In this pilot study, 5 µl of NM (0.02 mg/µL) was found to be optimal to simulate mustard gas injury on the ocular surface. PAC and CoQ10 doses were the same as those mentioned in previous studies(20-22). We irrigated the ocular surface with BSS and applied topical proparacaine hydrochloride (Alcaine 0.5%, Alcon Pharmaceuticals, Belgium) to relieve post-injury pain before reanimation.
Thirty minutes after the application of NM, the NM + PAC group received PAC (100 mg/kg) via gastric gavage, while the NM + CoQ10 group received CoQ10 (10 mg/kg) via i.p. injection. PAC and CoQ10 were administered once a day for 5 consecutive days. At the end of the 5th day, the animals were euthanized with cervical dislocation under anesthesia. They were examined under a slit-lamp bio-microscope, and images of the eyes and periocular tissues were obtained with a digital camera.
Macroscopic and histological evaluations
Macroscopic images of the eyes were examined for periocular and ocular structures. Corneal opacity was evaluated according to the grading system proposed by Sonoda and Streilein as follows: grade 0, totally transparent cornea; grade 1, minimal corneal opacity that allows the iris to be observed clearly; grade 2, mild corneal opacity and visible iris vessels; grade 3, moderate corneal opacity with invisible iris vessels and visible pupil margins; grade 4, total corneal opacity with no detectable anterior chamber structure(23,24). Lid injuries were also scored according to severity. In the literature, no previous study has evaluated chemical eyelid injuries or described a grading system for eyelid injuries. We scored lid injuries as follows: grade 0, total recovery and no visible lid injury; grade 1, mild superficial lesions without tissue defects; grade 2, moderate lid injury with some eyelash loss and local tissue defects; grade 3, total disruption of the lid margin and extreme loss of eyelash follicles or symblepharon formation (Table 1). At the end of the study, the eyes were enucleated, and they were fixed in formaldehyde for 24 h, stained with hematoxylin and eosin, and examined under a light microscope. The histological features of the corneal and lid tissues (cornea: epithelium and stromal structures; lid: epithelium, muscle fibers, secretory glands, distribution of inflammatory cells, and necrosis) were evaluated. A blinded ophthalmologist and a blinded histologist performed all of the evaluations.
Statistical analysis
Median, minimum, and maximum values are used to define the data. The Kruskal-Wallis test was used to compare multiple groups. For the post-hoc test, we selected the Mann-Whitney U test with Bonferroni correction. All analyses were performed using SPSS 22.0 (IBM Corp., Armonk, NY, USA). A p-value <0.05 was considered statistically significant.
RESULTS
Macroscopic results
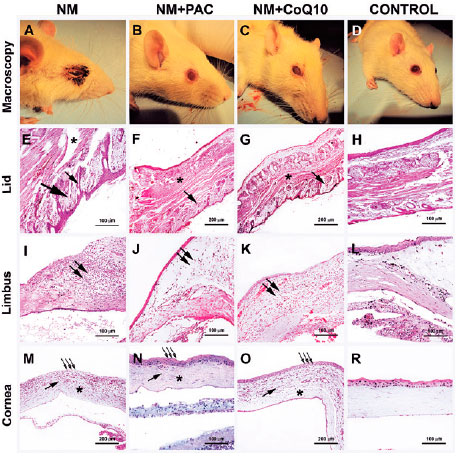
Mice that received NM showed corneal clouding, severe adnexal irritation, and deformation of eyelid margins (Figure 1A 1D). Our approach for triggering NM-induced ocular injury (appropriate for the research) was successful, as confirmed by comparison of the NM and control groups (p=0.048). The treatment groups were compared to the control group with regard to both corneal opacity and lid injury scores. The findings were not significantly different for both the NM + CoQ10 (p=0.33 for corneal opacity and p=0.318 for lid injury) and NM + PAC groups (p=0.93 for corneal opacity and p=0.95 for lid injury). These similarities between the treatment and control groups indicate that CoQ10 and PAC have therapeutic effects on NM-induced ocular injury. Although there were no significant differences between the NM + PAC and NM + CoQ10 groups, corneal opacity results appeared to be better in the NM + PAC group (p=0.33).
Histological results
In the NM group, all corneal sections showed epithelial desquamation, destruction of stromal fibers, stromal inflammatory cell infiltration, and limbal lymphocyte infiltration (Figure 1L, 1M). In addition, in the NM group, all lid sections showed degeneration of muscle fibers, deformation of hair follicles on the eyelashes, and loss of hypodermal connective tissue (Figure 1E). These histological changes are similar to those noted in acute alkaline burn of the ocular surface(25-27).
In the NM + PAC group, corneal sections revealed that PAC ameliorated the histological changes seen in the NM group (Figure 1J, 1N) and lid sections showed that muscle fibers of the eyelid and hair follicles of the eyelashes were intact (Figure 1F).
In the NM + CoQ10 group, it was found that CoQ10 attenuated the abnormal appearance of ocular tissues. Light microscopic investigation showed that treatment with CoQ10 improved the structure of the muscle fibers of the eyelid and hair follicles of the eyelashes (Figure 1G). Corneal epithelium and stromal fibers were also intact. Inflammatory cell infiltration in the stroma was present. Lymphocyte infiltration could be seen in the limbus (Figure 1K, 1O). The superficial epithelium of the eyelid was damaged in the NM group. In both NM + PAC and NM + CoQ10 groups, the epidermis of the eyelid was intact. None of the groups showed corneal neovascularization.
DISCUSSION
Oxidative stress and inflammation are considered critical factors in the course of NM toxicity(8,28). Zhou et al. identified a relationship between reactive oxygen species (ROSs) and inflammation, and this relationship suggested that ROSs derived from mitochondria act as signal transducing molecules to trigger pro-inflammatory cytokine production(29).
Kadar et al. reported that combined treatment with steroids and non-steroidal anti-inflammatory drugs following mustard exposure increased the anti-inflammatory effects of the drugs on ocular injury(30). However, inflammatory response reduction appeared insufficient to inhibit limbal destruction. As anti-inflammatory treatment alone may not always be satisfactory, both anti-inflammatory and antioxidant therapies should be considered to prevent delayed chronic inflammation. Banin et al. reported that metallocomplex eye drops resulted in significant improvements in NM-induced ocular injury because of their enhanced ability to inhibit transition metal-dependent formation of free radicals(27). We used PAC and CoQ10 as antioxidant agents in our study. Bagchi et al. reported that PAC provides significantly greater protection against free radicals and free radical-induced lipid peroxidation and DNA damage than vitamins C, E, and beta-carotene in both in vitro and in vivo models(31). In addition to the antioxidant activity of PAC, it has anti-inflammatory effects(20).
Wang et al. investigated the protective effects of CoQ10 on oxidative damage. The authors reported that CoQ10 treatment significantly decreased the number of apoptotic and necrotic cells and blocked apoptosis of human lens epithelial cells because of antioxidant activity(13). Wang et al. also revealed that administration of CoQ10 to lens epithelial cells significantly increased ATP levels. As ATP depletion has been reported to induce apoptosis, the protective function of CoQ10 can be attributed to this effect(13,32).
Ocular mustard exposure has been shown to cause not only local oxidative stress but also systemic oxidative stress(27,33). Therefore, the systemic antioxidant effects of PAC and CoQ10 treatments may contribute to the prevention of systemic oxidative stress following ocular NM exposure.
Thus far, the literature has not discussed a specific treatment option for mustard agent-related ocular injury. We performed the present study to investigate the effects of PAC and CoQ10 on NM-induced ocular injury. We designed an experimental model to evaluate the effects of PAC and CoQ10 on NM-induced ocular injury. Our results suggested that both PAC and CoQ10 treatments help the eye recover from NM-induced injury and corneal clouding. Further studies, including assessments of oxidative stress parameters and pro-inflammatory and anti-inflammatory markers, with a larger study group should be performed to determine the optimal treatment modalities in practice.
Our results indicate that systemic oral administrations of PAC and CoQ10 have therapeutic effects on NM-induced ocular injury in a rat model. PAC and CoQ10 may be novel treatment options in patients with NM-induced ocular injury. To our knowledge, this is the first study to present data about the protective effects of PAC and CoQ10 on NM-induced ocular injury. Based on our findings, we suggest that PAC and CoQ10 should be considered as dietary supplements for specific populations that have a high risk of chemical assault, such as soldiers.
REFERENCES
1. Naghii MR. Sulfur mustard intoxication, oxidative stress, and antioxidants. Mil Med. 2002;167(7):573-5.
2. Ghasemi H, Owlia P, Jalali-Nadoushan MR, Pourfarzam S, Azimi G, Yarmohammadi ME, et al. A clinicopathological approach to sulfur mustard-induced organ complications: a major review. Cutan Ocul Toxicol. 203;32(4):304-24.
3. Chemical casualties. Vesicants (blister agents). J R Army Med Corps. 2002;148(4):358-70.
4. Javadi MA, Jafarinasab MR, Feizi S, Karimian F, Negahban K. Management of mustard gas-induced limbal stem cell deficiency and keratitis. Ophthalmology. 2011;118(7):1272-81.
5. Kadar T, Cohen M, Cohen L, Fishbine E, Sahar R, Brandeis R, et al. Endothelial cell damage following sulfur mustard exposure in rabbits and its association with the delayed-onset ocular lesions. Cutan Ocul Toxicol. 2013;32(2):115-23.
6. Plahovinsak JL, Buccellato MA, Reid FM, Graham JS. Selection of non-steroidal anti-inflammatory drug and treatment regimen for sulfur mustard-induced cutaneous lesions. Cutan Ocul Toxicol. 2016; 35(3):208-17.
7. Balali-Mood M, Hefazi M. The pharmacology, toxicology, and medical treatment of sulphur mustard poisoning. Fundam Clin Pharmacol. 2005;19(3):297-315.
8. Tewari-Singh N, Jain AK, Inturi S, Ammar DA, Agarwal C, Tyagi P, et al. Silibinin, dexamethasone, and doxycycline as potential therapeutic agents for treating vesicant-inflicted ocular injuries. Toxicol Appl Pharmacol. 2012;264(1):23-31.
9. Amir A, Turetz J, Chapman S, Fishbeine E, Meshulam J, Sahar R, et al. Beneficial effects of topical anti-inflammatory drugs against sulfur mustard-induced ocular lesions in rabbits. J Appl Toxicol. 2000; 20(S1 Suppl 1):S109-14.
10. Turk A, Aykut M, Akyol N, Kola M, Mentese A, Sumer A, et al. Serum anti-carbonic anhydrase antibodies and oxidant-antioxidant balance in patients with acute anterior uveitis. Ocul Immunol Inflamm. 2014;22(2):127-32.
11. Kumar D, Tewari-Singh N, Agarwal C, Jain AK, Inturi S, Kant R, et al. Nitrogen Mustard Exposure of Murine Skin Induces DNA Damage, Oxidative Stress and Activation of Mapk/Akt-Ap1 Pathway Leading to Induction of Inflammatory and Proteolytic Mediators. Toxicol Lett. 2015;235:161-71.
12. Gueven N, Woolley K, Smith J. Border between natural product and drug: comparison of the related benzoquinones idebenone and coenzyme Q10. Redox Biol. 2015;4:289-95.
13. Wang S, Zhang J, Jiang T, Zheng L, Wang Z, Zhang J, et al. Protective effect of Coenzyme Q(10) against oxidative damage in human lens epithelial cells by novel ocular drug carriers. Int J Pharm. 2011; 403(1-2):219-29.
14. Tomasetti M, Alleva R, Borghi B, Collins AR. In vivo supplementation with coenzyme Q10 enhances the recovery of human lymphocytes from oxidative DNA damage. FASEB J. 2001;15(8):1425-7.
15. Fujisawa S, Kadoma Y. Kinetic study of the radical-scavenging activity of vitamin E and ubiquinone. In Vivo. 2005;19(6):1005-11.
16. Carletti G, Nervo G, Cattivelli L. Flavonoids and Melanins: a common strategy across two kingdoms. Int J Biol Sci. 2014;10(10): 1159-70.
17. Jain AK, Tewari-Singh N, Inturi S, Kumar D, Orlicky DJ, Agarwal C, et al. Flavanone silibinin treatment attenuates nitrogen mustard-induced toxic effects in mouse skin. Toxicol Appl Pharmacol. 2015; 285(1):71-8.
18. Nepote V, Maestri DM, Lamarque AL, Zygadlo JA. Natural products as antioxidants. In: Imperato F, editor. Phytochemistry: advances in research. Kerala, India: Research Signpost; 2006. p. 105-35.
19. Li WG, Zhang XY, Wu YJ, Tian X. Anti-inflammatory effect and mechanism of proanthocyanidins from grape seeds. Acta Pharmacol Sin. 2001;22(12):1117-20.
20. Yucel O, Genc O, Onguru O, Aydýn A, Þahin MA, Güler A, et al. Proanthocyanidine alleviates lung damage induced by nitrogen mustard. Gulhane Tip Derg. 2008;50:267-72.
21. Baskaran UL, Sabina EP. The food supplement coenzyme Q10 and suppression of antitubercular drug-induced hepatic injury in rats: the role of antioxidant defence system, anti-inflammatory cytokine IL-10. Cell Biol Toxicol. 2015;31(4-5):211-9.
22. Gokce M, Saydam O, Hanci V, Can M, Bahadir B. Antioxidant vitamins C, E and coenzyme Q10 vs dexamethasone: comparisons of their effects in pulmonary contusion model. J Cardiothorac Surg. 2012;7(1):92.
23. Sonoda Y, Streilein JW. Impaired cell-mediated immunity in mice bearing healthy orthotopic corneal allografts. J Immunol. 1993; 150(5):1727-34.
24. Ke Y, Wu Y, Cui X, Liu X, Yu M, Yang C, et al. Polysaccharide hydrogel combined with mesenchymal stem cells promotes the healing of corneal alkali burn in rats. PLoS One. 2015;10(3):e0119725.
25. Espandar L, Caldwell D, Watson R, Blanco-Mezquita T, Zhang S, Bunnell B. Application of adipose-derived stem cells on scleral contact lens carrier in an animal model of severe acute alkaline burn. Eye Contact Lens. 2014;40(4):243-7.
26. Lin HF, Lai YC, Tai CF, Tsai JL, Hsu HC, Hsu RF, et al. Effects of cultured human adipose-derived stem cells transplantation on rabbit cornea regeneration after alkaline chemical burn. Kaohsiung J Med Sci. 2013;29(1):14-8.
27. Banin E, Morad Y, Berenshtein E, Obolensky A, Yahalom C, Goldich J, et al. Injury induced by chemical warfare agents: characterization and treatment of ocular tissues exposed to nitrogen mustard. Invest Ophthalmol Vis Sci. 2003;44(7):2966-72.
28. Korkmaz A, Yaren H, Kunak ZI, Uysal B, Kurt B, Topal T, et al. Epigenetic perturbations in the pathogenesis of mustard toxicity; hypothesis and preliminary results. Interdiscip Toxicol. 2008;1(3-4): 236-41.
29. Zhou R, Yazdi AS, Menu P, Tschopp J. A role for mitochondria in NLRP3 inflammasome activation. Nature. 2011;469(7329):221-5.
30. Kadar T, Dachir S, Cohen L, Sahar R, Fishbine E, Cohen M, et al. Ocular injuries following sulfur mustard exposure-pathological mechanism and potential therapy. Toxicology. 2009;263(1):59-69.
31. Bagchi D, Bagchi M, Stohs SJ, Das DK, Ray SD, Kuszynski CA, et al. Free radicals and grape seed proanthocyanidin extract: importance in human health and disease prevention. Toxicology. 2000;148(2-3): 187-97.
32. Nakamura N, Wada Y. Properties of DNA fragmentation activity generated by ATP depletion. Cell Death Differ. 2000;7(5):477-84.
33. Kadar T, Turetz J, Fishbine E, Sahar R, Chapman S, Amir A. Characterization of acute and delayed ocular lesions induced by sulfur mustard in rabbits. Curr Eye Res. 2001;22(1):42-53.
Submitted for publication:
June 20, 2017.
Accepted for publication:
January 8, 2018.
Approved by the following research ethics committee: Gulhane Military Medical Academy (#AR-GE:8200-1090-10/1594)
Funding: No specific financial support was available for this study
Disclosure of potential conflicts of interest: The authors have no potential conflicts of interest to disclose